The TiF Checklist
The checklist can be viewed in two ways. You can either view the annotated checklist on these web pages or download a list. The downloadable lists are Excel csv files that can be imported into spreadsheets such as Excel, or easily manipulated by programs such as perl. Four lists are available in csv format. The ABA list includes only ABA recognized species, but in TiF order, with TiF families. The South American list uses TiF species rather than SACC species.
Further, Stephen Nawrocki has provided an excel version of the worldlist, version 2.79.
You can view an annotated version by clicking on the list of bird orders on the right, or by going to the family index, or by clicking on the family names in the various tree view pages. In the annotated list, recently extinct species and species whose taxonomic placement is particularly uncertain are color-coded. In some cases, superfamilies, subfamilies, tribes, and other groups have been added to help show how the birds are related.
The 46 Orders
The TiF checklist currently groups the birds in 46 Orders and 249 families. Both a order-level and family-level trees are now availalbe in pdf format. Due to its length, the family tree is split into 5 parts.
 |
 |
| 46 Orders | 249 Families |
|---|
The 46 orders of the TiF list include a few more orders than most other lists. However, I wanted to make sure that each of the orders was monophyletic, meaning that they include all descendants of a common ancestor. I did not have confidence that a shorter list would do. As it currently stands, I am highly confident that each order is monophyletic.
The arrangement of the orders is primarily based on Jarvis et al. (2014). Previous versions were based on Hackett et al. (2008) and Gibb et al. (2013), or even earlier Ericson et al. (2006a). Jarvis et al. (2014) have the deepest coverage, analyzing data taken from 8251 genes and 3769 non-overlapping ultra conserved elements, totalling about 41.8 million base pairs (bp), about 3.5% of the avian genome. In contrast, the previous effort by Hackett et al. used about 31,000 bp from 19 genes, while the earlier paper by Ericson et al. (2006a) relied on about 5000 bp from 5 genes.
Supra-ordinal Groups
Not all of the Jarvis tree is equally reliable. We can be pretty confident that 9 of the major supra-ordinal groups are monophyletic, as are the four subgroups listed below. Moreover, we have a good idea of how the orders within each group relate, and their family structure. There is still a bit of uncertainty regarding relationships between the supra-ordinal groups. These groups are:
Jarvis et al. (2014) only included taxa from 39 orders in their analysis, omitting 3 of the Paleognathae, 3 of the Strisores, and Ciconiiformes. Hackett et al. found strong support for the Paleognaths (as have many other analyses). Indeed, that they all posessed a distinctive palate was already recognized by Huxley (1867). Hackett et al. (2008) also found good support (98% bootstrap) for the Strisores, and placed the Ciconiformes well inside the Aequornithes (as do most other analyses).
PALEOGNATHAE
STRUTHIONIFORMES
RHEIFORMES
CASUARIIFORMES
APTERYGIFORMES
TINAMIFORMES
GALLOANSERAE
ANSERIFORMES
GALLIFORMES
COLUMBEA
MIRANDORNITHES
PHOENICOPTERIFORMES
PODICIPEDIFORMES
COLUMBIMORPHAE
MESITORNITHIFORMES
PTEROCLIFORMES
COLUMBIFORMES
OTIDIMORPHAE
MUSOPHAGIFORMES
OTIDIFORMES
CUCULIFORMES
STRISORES
STEATORNITHIFORMES
NYCTIBIIFORMES
PODARGIFORMES
CAPRIMULGIFORMES
APODIFORMES
GRUIMORPHAE
GRUIFORMES
CHARADRIIFORMES
EURYPYGIMORPHAE
EURYPYGIFORMES
PHAETHONTIFORMES
AEQUORNITHES
GAVIIFORMES
SPHENISCIFORMES
PROCELLARIIFORMES
CICONIIFORMES
SULIFORMES
PLATALEIFORMES
PELECANIFORMES
ARDEIFORMES
TELLURAVES
AFROAVES
CATHARTIFORMES
ACCIPITRIFORMES
STRIGIFORMES
COLIIFORMES
LEPTOSOMIFORMES
TROGONIFORMES
BUCEROTIFORMES
CORACIIFORMES
PICIFORMES
AUSTRALAVES
CARIAMIFORMES
FALCONIFORMES
PSITTACIFORMES
PASSERIFORMES
The Hoatzin Problem
If you are counting, you will have noticed that only 45 of the orders are listed above. The missing order is Opisthocomiformes. It contains a single species, the Hoatzin. Mostly likely, it is sister to the Gruiformes, but support for this is rather weaker than for the groups listed above. Indeed, except for the Gruiformes (96%), all of the supra-ordinal groups given above had 100% bootstrap support. In the case of the Gruimorphae, there has long been morphological evidence linking the Gruiformes and Charadriiformes.
High-level Avian Relationships
The deepest division in the avian tree is between the Paleognathae and everything else (the Neognathae). It has been known for some time that the next division is between Galloanserae and the rest (Neoaves), at least since Sibley and Ahlquist (1990).
The relationships within Neoaves have been more problematic. This is likely because the splits of Paleognathae and Galloanserae occured before the bolide crashed into Chicxulub to end the Cretaceous (66.04 million years ago, see Renne et al., 2013). The surviving birds rapidly diversified following the impact, creating problems such as incomplete lineage sorting at supra-ordinal levels within Neoaves. Untangling this has been a protracted process. It is now twenty-five years after Sibley and Ahlquist, and the problem has still not been completely solved. Jarvis et al. (2014) threw a huge amount of data at the problem, three orders of magnitude more depth than Hackett et al. (2008). Further increases in depth will not be as large, and I suspect that further adjustments to the higher-order phylogeny will be rather modest, with gains coming as much from increased breadth of sampling as from increased depth.
Jarvis et al. found that Neoaves divides into 2 groups, Columbea and Passerea. This puts an end to Fain and Houde's (2004) Metaves hypothesis, whether in the original form, or in that of Ericsson et al. (2006a) or Hackett et al. (2008). Columbea is a fragment of Metaves. There were hints in Ericson et al. (Figures ESM-6 and ESM-7). Columbea is comprised of two groups: Mirandornithes and Columbimorphae. One of the surprises of the 2000's was the close realtion of the grebes and flamingos (Mirandornithes), and it has held up. Another surprise was grouping the Mesites near the pigeons. This too has held up.
The Passerea are still something of a problem. We know there's a big group buried in it, the higher land birds (including raptors), Telluraves. However, the branching order from the base of Passerea to Telluraves remains inadequately resolved.
The Jarvis et al. (2014) tree has the Otidae (Otidimorphae and Strisores) as the basal branch, followed by Gruae (Gruimorphae and the always troublesome Hoatzin). The rest then divide into the waterbird clade Ardeae (Eurypygimorphae and Aequornithes) and Telluraves. Evidence for each of the intermediate branches from Otidae to Ardae is a little soft, although support for the components seems to be good, as does support for Telluraves itself.
PALEOGNATHAE
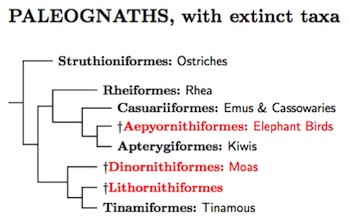 |
The first major division among the living birds is between the Paleognathae and Neognathae. The taxonomy of the Paleognathae continues to be controversial. Traditionally, they have been divided into the flightless ratites and the volant tinamous. Some of the earlier generic evidence seemed to support this (e.g., Haddrath and Baker, 2001).
However, the recent studies of Chojnowski et al. (2008), Hackett et al. (2008), Harshman et al. (2008), and Phillips et al. (2009), Faircloth et al. (2012), Haddrath and Baker (2012), and J.V. Smith et al. (2013) have come to a very different conclusion. The ratites are not monophyletic. Some of the them are more closely related to the volant tinamous than to other ratites. In particular, the ostriches are no more closely related to the other ratites than to the tinamous, and the other ratites share a more recent common ancestor with the tinamous than with the ostriches.
Once we get beyond the ostriches, things are not so clear-cut. There are three major groups: (1) rheas, (2) tinamous (and the recently extinct moas), and (3) cassowaries, emus, and kiwis. The three groups seem to have separated over a short period of time. The next question is which of the three groups separated first?
Currently, this has not been conclusively resolved. However, it appears that it is either the rheas or the tinamou/moa clade (see J.V. Smith et al., 2013). Many analyses (e.g., Harshman et al., 2008; Phillips et al., 2009; J.V. Smith et al., 2013) favor putting the rheas first. However, when Haddrath and Baker (2012) considered retroposons, the balance shifted to the tinamaou/moa clade, and that is the topology we will follow here.
This topology implies that flightlessness has evolved at least 3 times in the Paleognathae: in ostriches, in moas, and at least once among the rheas, emus, cassowaries, and kiwis. Indeed, if the chronogram in Haddrath and Baker (2012) is correct, both the split of the rheas and Austalasian ratites and the split between the kiwis and emus/cassowaries occurred about 80 million years ago (mya). This timing is consistent with the kiwis originating due to the separation of Australia and New Zealand and with the ancestral rheas walking their way across the then-temperate Antarctica to South America.
There is further relevant DNA evidence indicating that the extinct elephant birds of Madagascar are the closest relatives of the kiwis (Mitchell et al., 2014b), with a common ancestor perhaps 50 mya. This is a problem for vicariance theories because Madagascar (and India) likely separated from Gondwana considerably earlier, perhaps 120 mya. In that case, the ancestor of the elephant birds would have had to fly, and we can infer that flight was indpendently lost in the rheas, emu/cassorwary, elephant bird, and kiwi lineages, a total of 6 losses of flight. This is not an unreasonable number. One only has to consider all of the flightless rails to see that.
The fossil evidence considered by Johnston (2011) suggests that the long-extinct Lithornithiformes were close relatives of the tinamous.
The Paleognathae are divided into several orders in recognition of the great antiquity of the various branches, some probably dating back to the Cretaceous period. Phillips et al. (2009) estimate the ostriches diverged from the rest of the Paleognathae about 60-95 mya, while Chojnowski et al. (2008) date this divergence around 64±22 mya, near the K/T (=K/Pg) boundary. Haddrath and Baker (2012) put it at 73-119 mya. Finally, mapping Jarvis et al. (2014) onto the phylogeny used here suggests that the split was about 84 mya (interval 58-96 mya).
The Cassowaries and Emus seem much more closely related than the other paleognath families, and so are placed in a single order: Casuariiformes. In fact, the molecular dates in Phillips et al. and in Haddrath and Baker suggest they are so closely related that they could be treated as a single family. Although the Moas and Elephant Birds are not part of the main TiF list, a genus-level phylogeny of both is given in the tree diagram. The Moa tree is based on Bunce et al. (2009).
STRUTHIONIFORMES Latham, 1790
Struthionidae: Ostriches Vigors, 1825
The authors for family-group names are mostly based on Bock (1994), while the authors for order-group names are primarily based on the series by Brodkorb (1963-1978). In both cases, additional sources have been consulted (e.g., Livezey and Zusi (2007)). In a number of cases, I have managed to examine the original (thank you Google Books!). The ICZN does not regulate order-group names. However, for parvorders (-ida), infraorders (-ides), suborders (-i), orders (-iformes), and superorders (-imorphae), I am attempting to follow similar rules. In particular, they are based on priority and the orginal use must been at an ordinal level and must be based on an included genus name (type genus) actually used by that author. The endings have been adjusted to modern usage.
1 genus, 2 species HBW-1
- Common Ostrich, Struthio camelus
- Somali Ostrich, Struthio molybdophanes
RHEIFORMES Forbes, 1884
Rheidae: Rheas Bonaparte, 1849
1 genus, 2 species HBW-1
- Greater Rhea, Rhea americana
- Lesser Rhea, Rhea pennata
CASUARIIFORMES P.L. Sclater 1880
Dromaiidae: Emus Huxley, 1868
1 genus, 1 species HBW-1
Heupink et al. (2011) argue that the extinct King Island Emu, Dromaius ater, was quite closely related to the extinct Tasmanian subspecies of the Emu, and is best considered a dwarf subspecies of Dromaius novaehollandiae. The Kangaroo Island Emu, D. baudinianus, seems likely to have been no more different from the Emu than the King Island Emu was, so I am also considering it a subspecies of the Emu, D. novaehollandiae.
- Emu, Dromaius novaehollandiae
Casuariidae: Cassowaries Kaup, 1847
1 genus, 3 species HBW-1
- Southern Cassowary, Casuarius casuarius
- Dwarf Cassowary, Casuarius bennetti
- Northern Cassowary, Casuarius unappendiculatus
APTERYGIFORMES Haeckel 1866
The order of the Kiwis reflects the phylogeny in Burbidge et al. (2003) and Shepherd et al. (2012). Burbidge et al. also provide evidence for recognizing 3 extant species of brown kiwi, as is done here. Shepherd et al. found evidence of an extinct northern clade of Little Spotted Kiwi that may deserve recognition as a separate species. The DNA samples of this potential species are from bones of unknown age.
Apterygidae: Kiwis G.R. Gray, 1840
1 genus, 5 species HBW-1
- Little Spotted Kiwi, Apteryx owenii
- Great Spotted Kiwi, Apteryx haastii
- Southern Brown Kiwi, Apteryx australis
- North Island Brown Kiwi, Apteryx mantelli
- Okarito Kiwi, Apteryx rowi
TINAMIFORMES Huxley, 1872
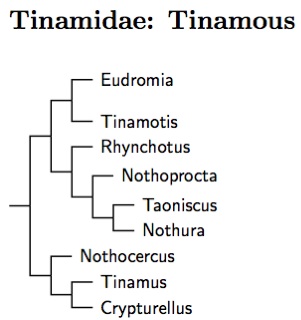 |
The Tinamiformes are the other branch of the Paleognathae. There is only one family. The taxonomy here is based on Bertelli and Porzecanski (2004) and SACC.
The Tinamidae are sometimes divided into two subfamilies: Rynchotinae and Tinaminae. This is consistent with the phylogeny shown, although there is some uncertainty about whether Nothocercus really groups with Tinamus and Crypturellus.
Tinamidae: Tinamous G.R. Gray, 1840
9 genera, 47 species HBW-1
- Elegant Crested-Tinamou, Eudromia elegans
- Quebracho Crested-Tinamou, Eudromia formosa
- Puna Tinamou, Tinamotis pentlandii
- Patagonian Tinamou, Tinamotis ingoufi
- Red-winged Tinamou, Rhynchotus rufescens
- Huayco Tinamou, Rhynchotus maculicollis
- Taczanowski's Tinamou, Nothoprocta taczanowskii
- Ornate Tinamou, Nothoprocta ornata
- Chilean Tinamou, Nothoprocta perdicaria
- Brushland Tinamou, Nothoprocta cinerascens
- Andean Tinamou, Nothoprocta pentlandii
- Curve-billed Tinamou, Nothoprocta curvirostris
- Dwarf Tinamou, Taoniscus nanus
- White-bellied Nothura, Nothura boraquira
- Lesser Nothura, Nothura minor
- Darwin's Nothura, Nothura darwinii
- Spotted Nothura, Nothura maculosa
- Chaco Nothura, Nothura chacoensis
- Tawny-breasted Tinamou, Nothocercus julius
- Highland Tinamou, Nothocercus bonapartei
- Hooded Tinamou, Nothocercus nigrocapillus
- Gray Tinamou, Tinamus tao
- Solitary Tinamou, Tinamus solitarius
- Black Tinamou, Tinamus osgoodi
- Great Tinamou, Tinamus major
- White-throated Tinamou, Tinamus guttatus
- Cinereous Tinamou, Crypturellus cinereus
- Berlepsch's Tinamou, Crypturellus berlepschi
- Little Tinamou, Crypturellus soui
- Tepui Tinamou, Crypturellus ptaritepui
- Brown Tinamou, Crypturellus obsoletus
- Undulated Tinamou, Crypturellus undulatus
- Pale-browed Tinamou, Crypturellus transfasciatus
- Brazilian Tinamou, Crypturellus strigulosus
- Gray-legged Tinamou, Crypturellus duidae
- Red-legged Tinamou, Crypturellus erythropus
- Yellow-legged Tinamou, Crypturellus noctivagus
- Black-capped Tinamou, Crypturellus atrocapillus
- Thicket Tinamou, Crypturellus cinnamomeus
- Slaty-breasted Tinamou, Crypturellus boucardi
- Choco Tinamou, Crypturellus kerriae
- Variegated Tinamou, Crypturellus variegatus
- Rusty Tinamou, Crypturellus brevirostris
- Bartlett's Tinamou, Crypturellus bartletti
- Small-billed Tinamou, Crypturellus parvirostris
- Barred Tinamou, Crypturellus casiquiare
- Tataupa Tinamou, Crypturellus tataupa